What is Alzheimer’s Disease?
The deposition of amyloid-β (Aβ) protein, which forms the core of extracellular amyloid plaques (senile plaques) implicated in neurodegenerative processes.
The accumulation of hyperphosphorylated tau protein, which constitutes intracellular neurofibrillary tangles and contributes to neuronal dysfunction in neurodegenerative disorders.
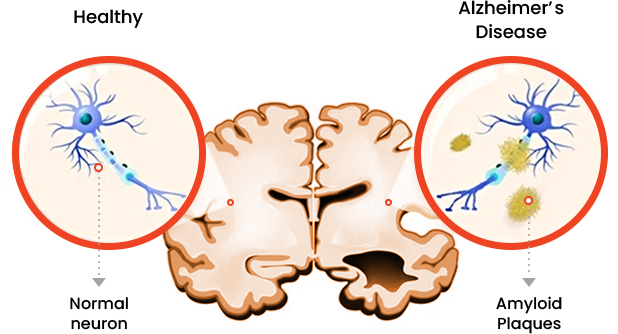

 The aggregation of amyloid-β and
hyperphosphorylated tau proteins is
a hallmark observed in the brains of
individuals with Alzheimer's disease.
The aggregation of amyloid-β and
hyperphosphorylated tau proteins is
a hallmark observed in the brains of
individuals with Alzheimer's disease.
Most Alzheimer’s drugs today only ease symptoms. They can’t stop the disease or address the root causes.
We need real solutions treatments that slow or stop brain damage and help restore thinking and memory.
Prevalence and Market Size Domestically
and Internationally
References : US Census Bureau, Alzheimers Association, etc.
- Prevalence
-
Among dementia patients aged 65 and older, approximately 70% are diagnosed with Alzheimer's disease-related dementia.
The prevalence of dementia among individuals aged 65 and older in South Korea is approximately 10.33% as of 2021.
The global prevalence of dementia among individuals aged 65 and older is approximately 10.7%.
- Market Size
-
The U.S. Market is Projected to Reach Approximately $129 Billion by 2050
The Global Market is Projected to Reach Approximately $2,688 Billion by 2050
Projected Number of Alzheimer’s Patients by Country
References : Johns Hopkins Medicine Inhealth, homepage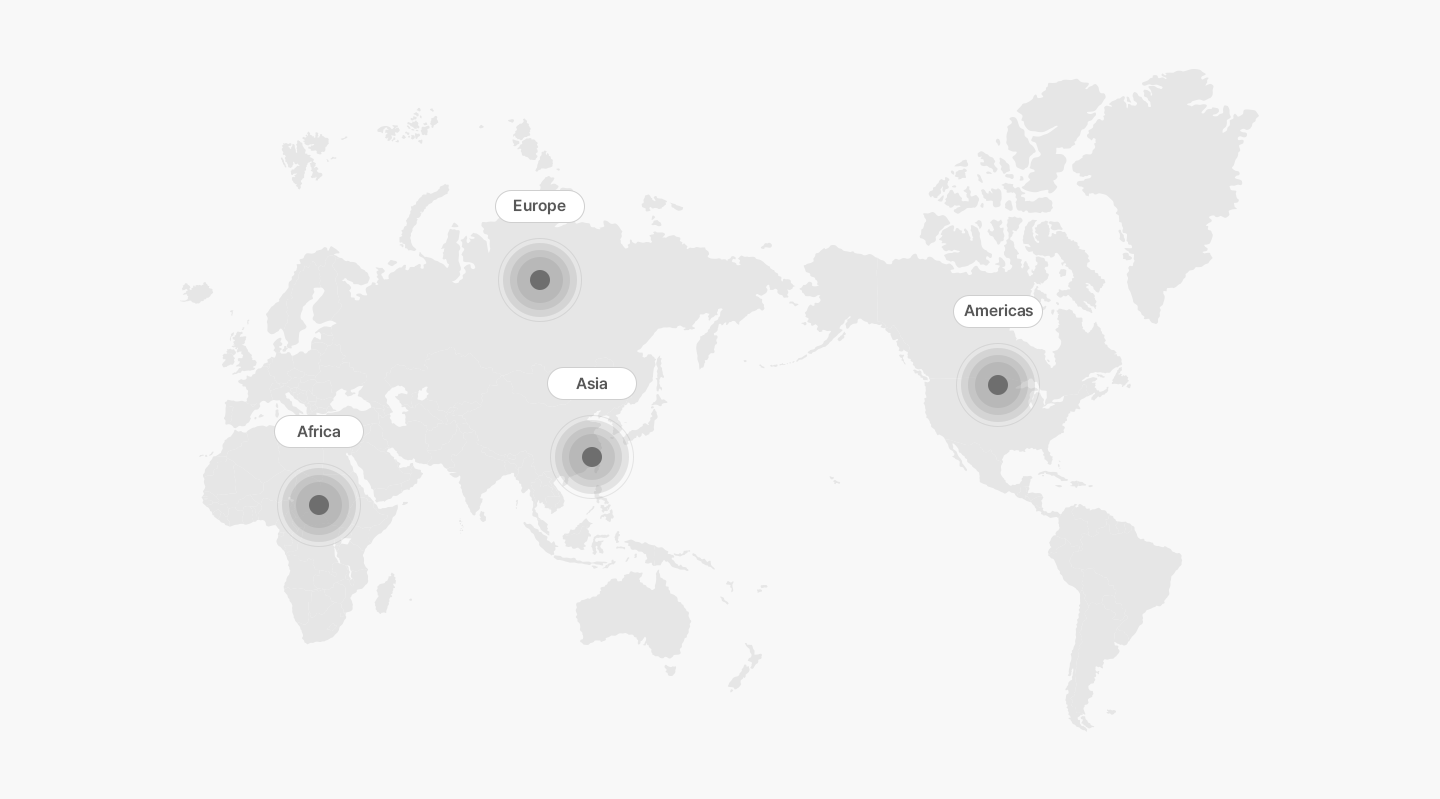
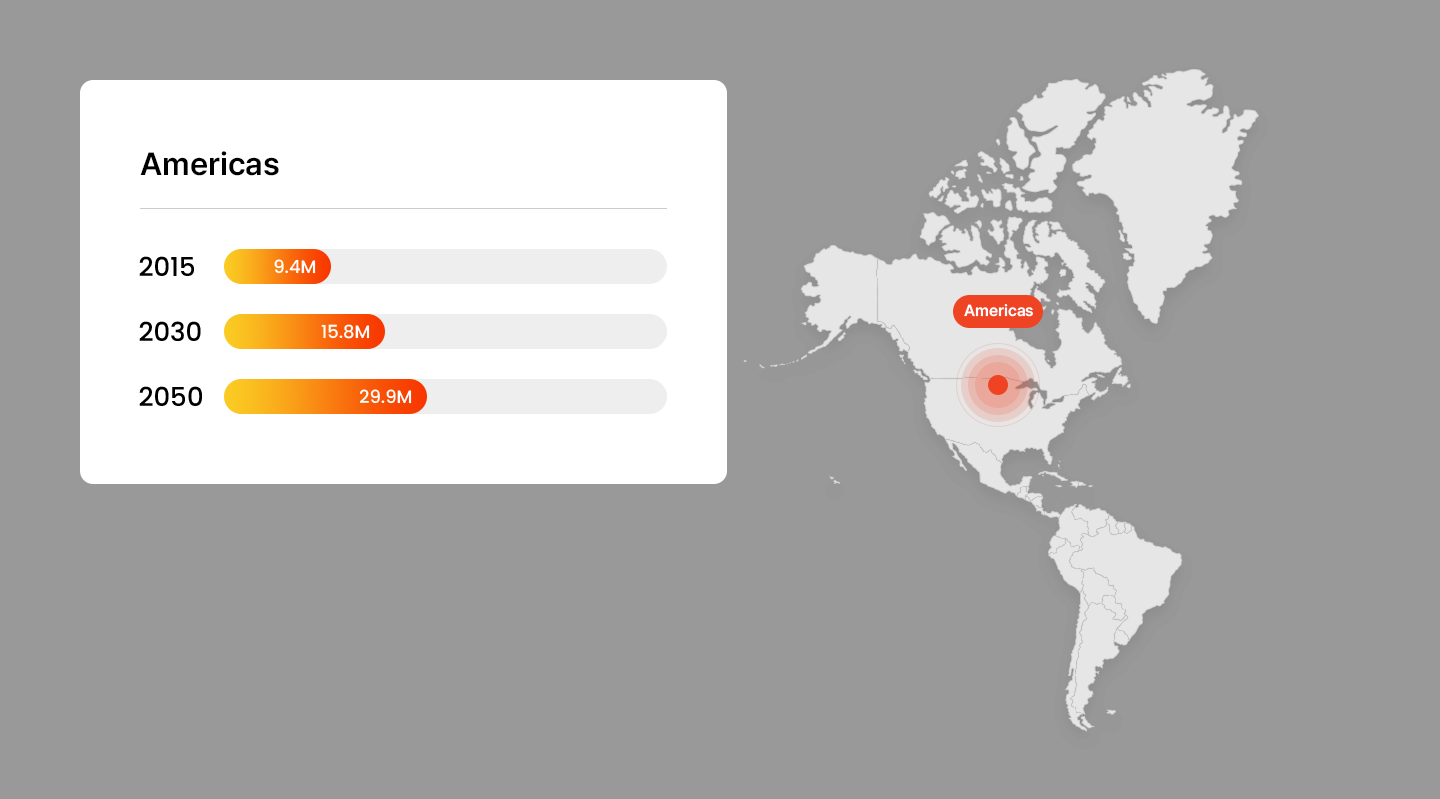
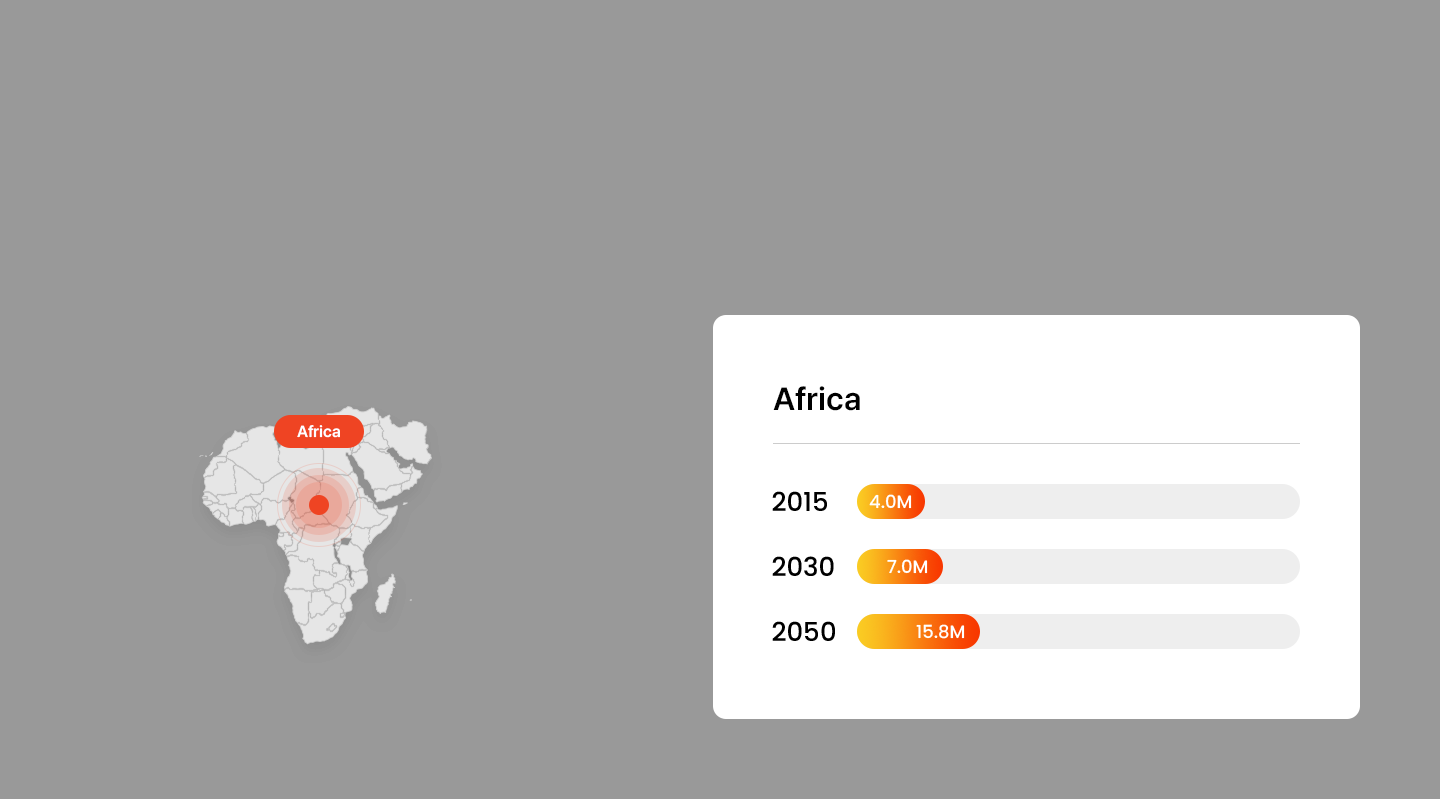
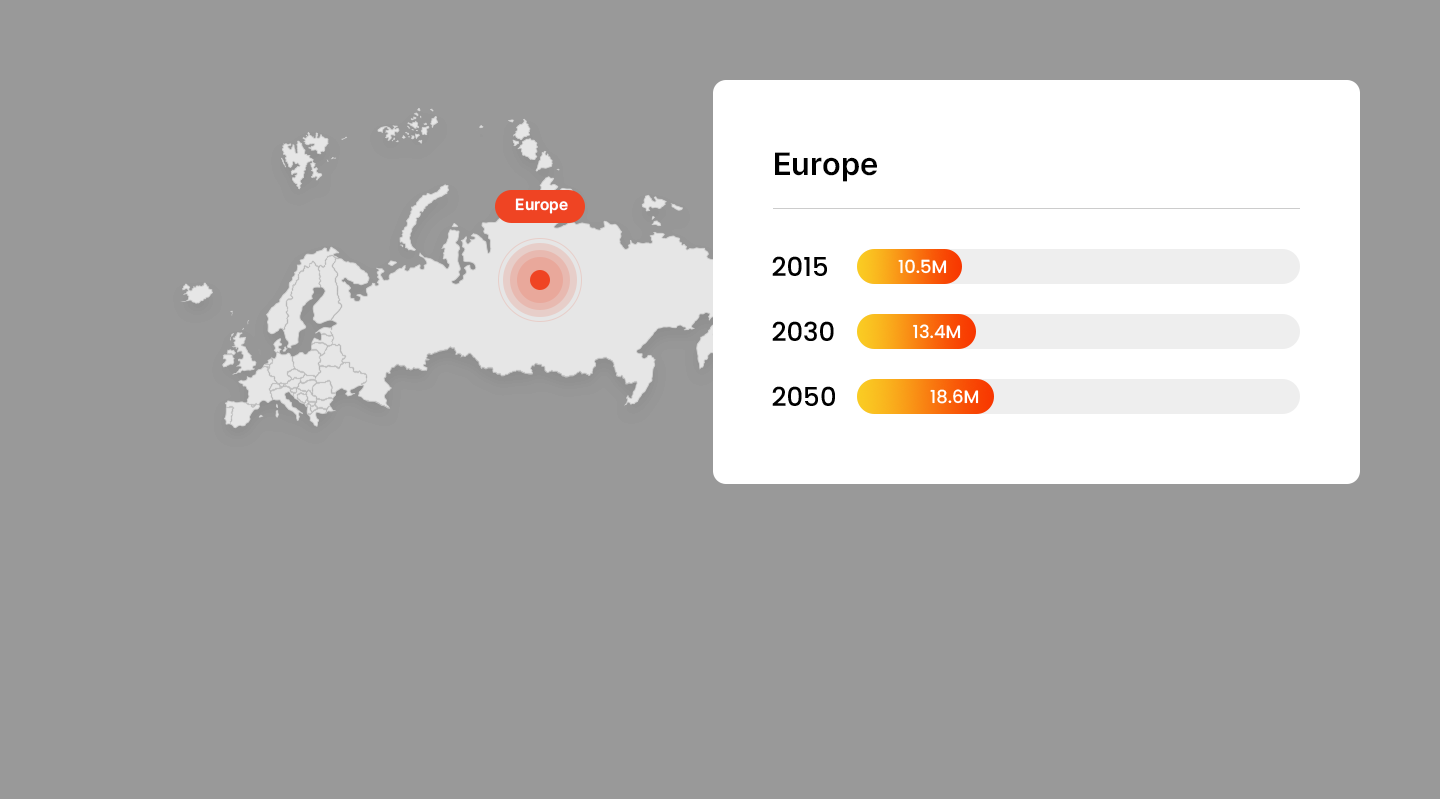
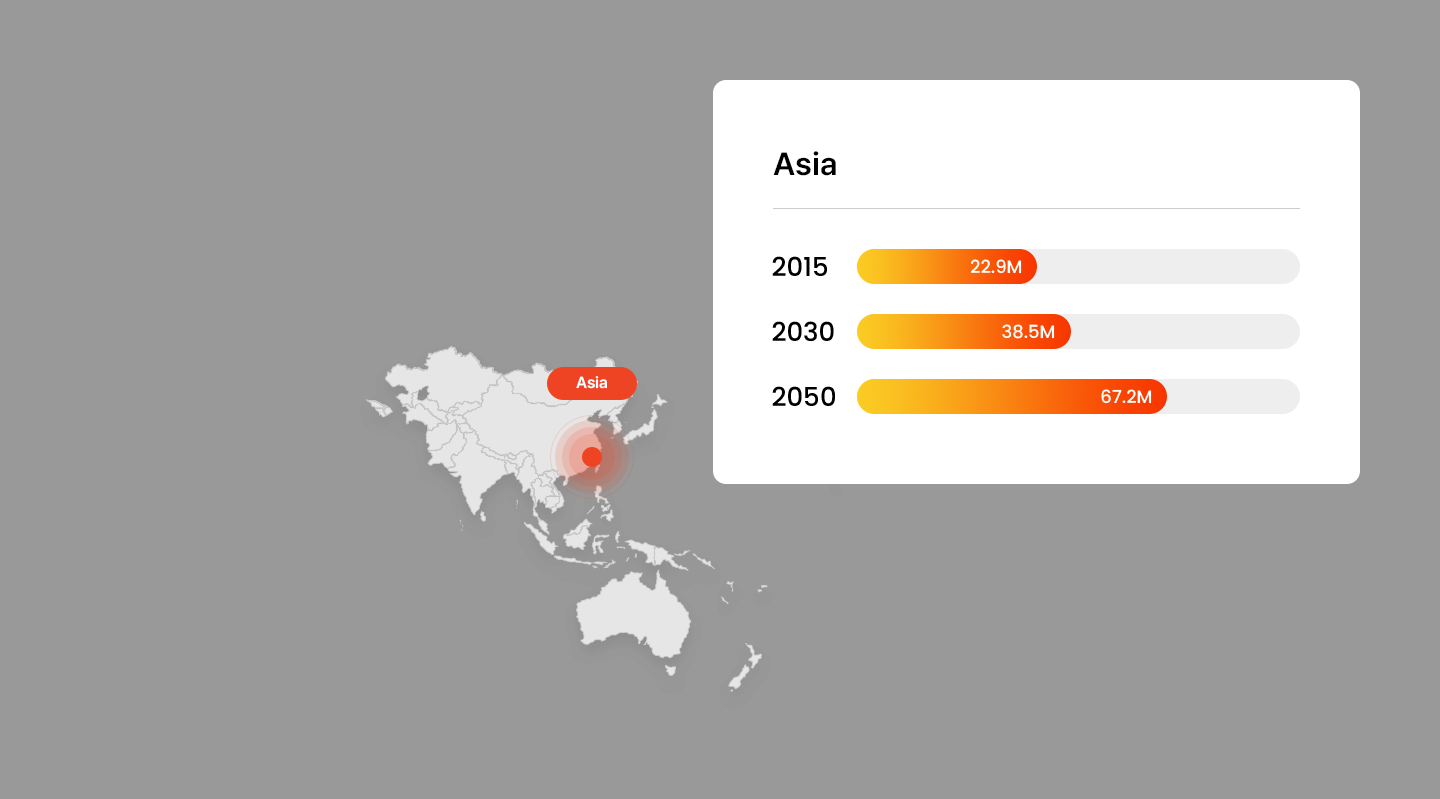
- America
- Asia
- Africa
- Europe
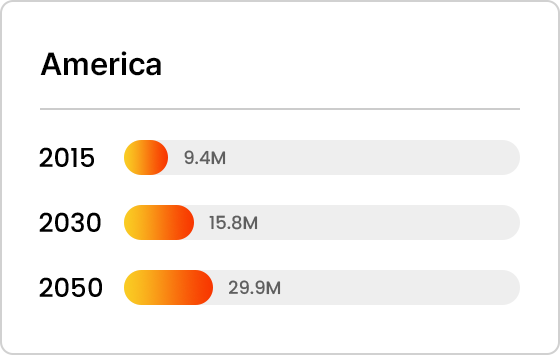
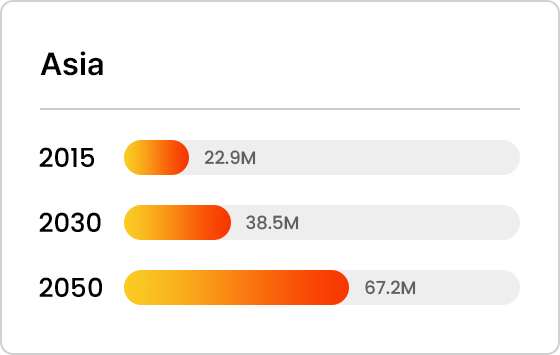
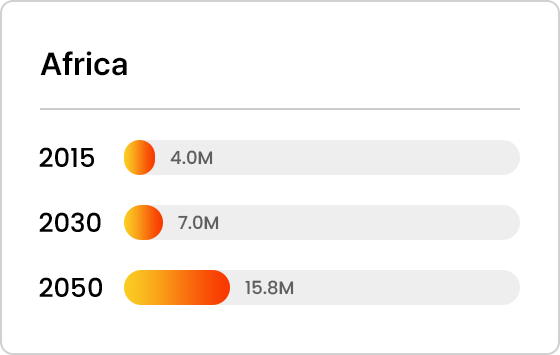
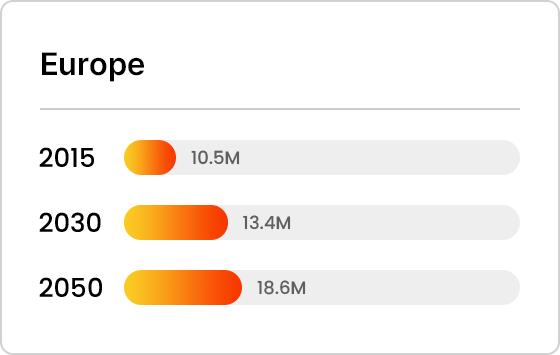
Trends in Alzheimer’s Patients Worldwide

Worldwide Distribution of Dementia Types

A New Era of Alzheimer’s Therapeutics
Unparalleled Innovation and Excellence in Dual-Target Technology
| Function | Development drug | Development status |
|---|---|---|
| Decreased Aβ plaque |
Aducanumab (Biogen) | Conditional Approval(2021) Withdraw(2023) |
| Lecanemab (Biogen-Eisai) | Approval(2023) | |
| Gantenerumab (Roche) | Phase 3 Clinical Trial Failure | |
| Donanemab (Eli Lilly) | Approval(2024) | |
| Reduced Hyper- phosphorylated Tau plaque |
LMTX (TauRx) | Phase 3 Clinical Trial Active |
| Verubecestat (Merck) | Phase 3 Clinical Trial Failure |
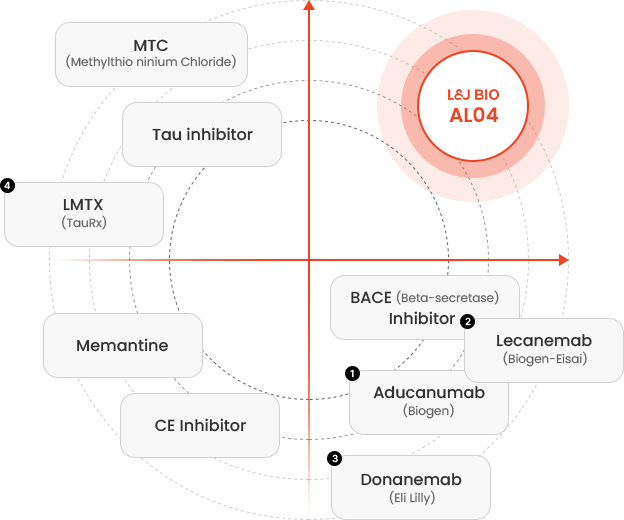
What is AL04?
L&J BIO’s lead product candidate-AL04 is targeting both Amyloid- β and p-Tau
- Dual Action Therapy Platform
-
Decrease Amyloid beta (Aβ) plaqueDecrease hyperphosphorylated Tau tangle
- Fusion Therapeutic Protein
-
Human Serum Albumin fusion protein: Extend half-life in the blood and expect sustainability
- Blood-Brain Barrier (BBB) Penetration
-
Using Cell Penetrate Peptide (CPP) for penetrating the BBB
Dual Action Therapy Platform
AL04 targets Amyloid β aggregation and Hyperphosphorylated Tau
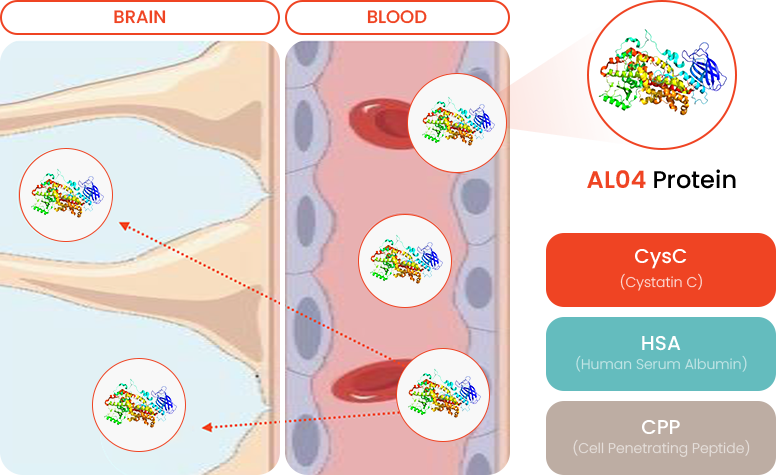
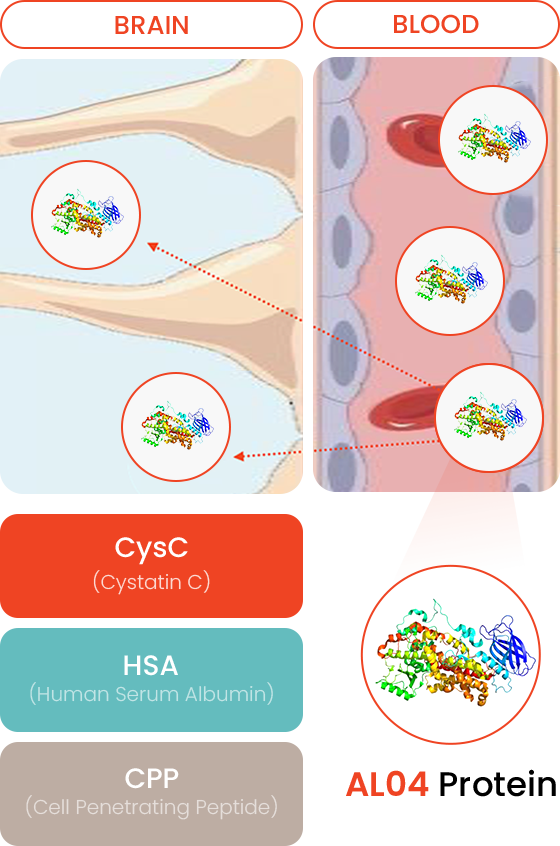
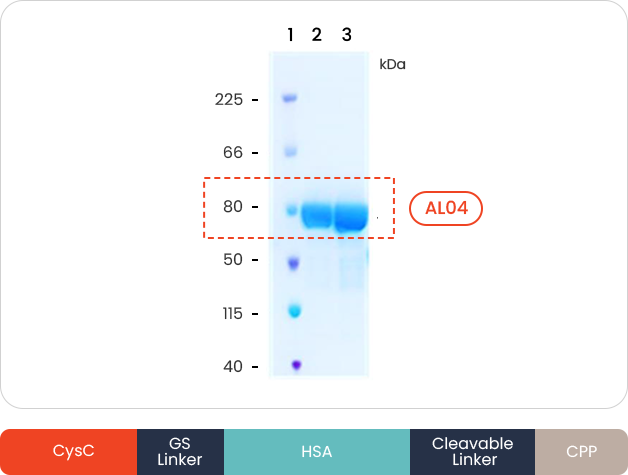
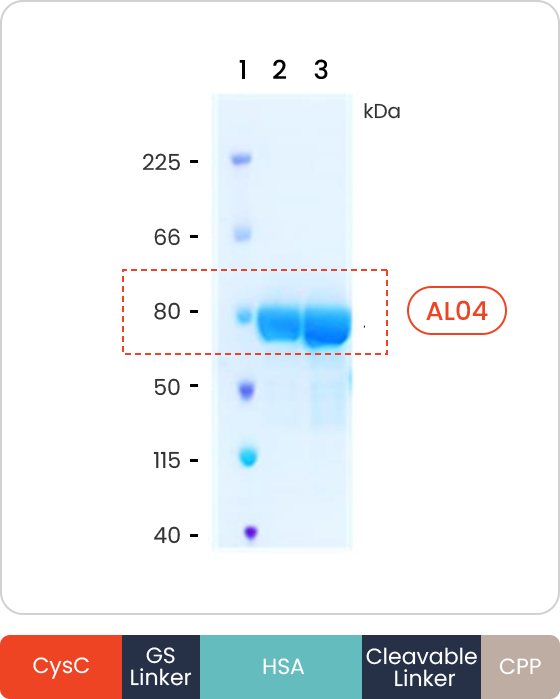
- CysC (Cystatin C)
-
Reduction of Amyloid β plague
Decreasing hyperphosphorylated Tau (Neurofibrillary tangle)
- HSA (Human Serum Albumin)
-
Increasing half-life and sustainability
- CPP (Cell Penetrating Peptide)
-
Newly designed BBB penetrable CPP (Cell Penetrating Peptide)
AL04 Decreases the Aβ Accumulations in the Brain
Tg2576 mice : over-expressed human APP (Amyloid Precursor Protein) with the Swedish double mutations (K670N, M671L)
Amyloid Beta (Aβ) Target
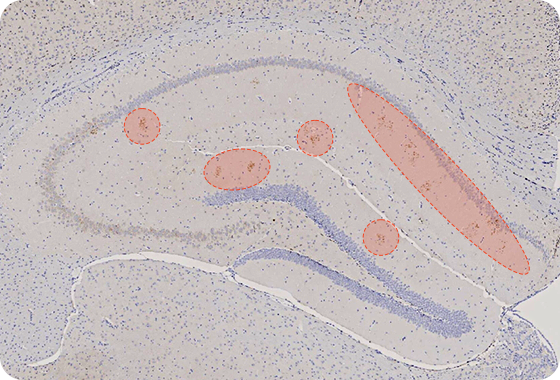
Aβ is accumulated in mouse brain
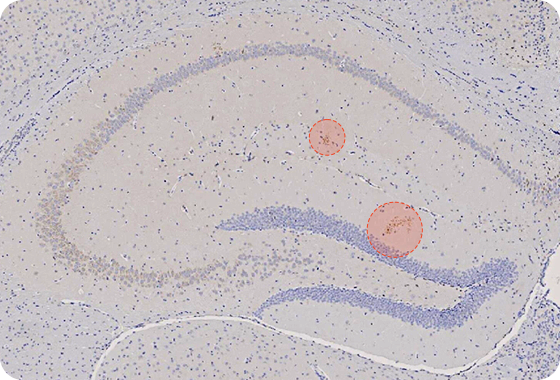
Amount of Aβ is reduced in mouse brain
Reducing amount of Aβ in Hippocampus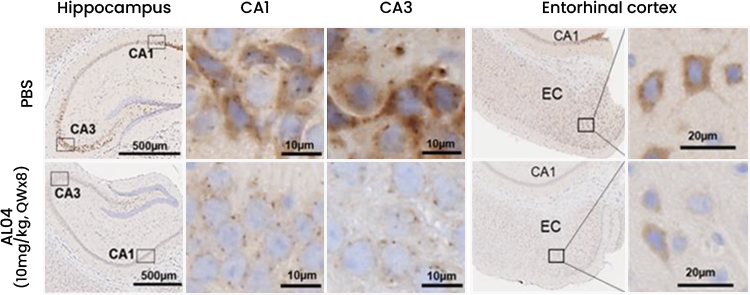
- AL04 decrease the Aβ accumulations in the hippocampus and the entorhinal cortex
- AL04 attenuates neuron associated the accumulation of Aβ in the hippocampus

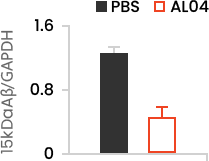
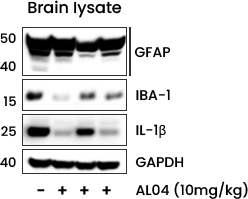
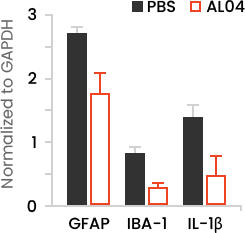
- AL04 can reduce the neuro-inflammatory damage in association with the Aβ deposition
AL04 Decreases Hyperphosphorylated Tau Tangles
JNPL3 mice : hyperphosphorylated Tau accumulation model, administered with AL04 at a concentration of 5 mg/kg by intraperitoneal injection at 2-week intervals for 6 months.
phosphorylated Tau (p-Tau) Target
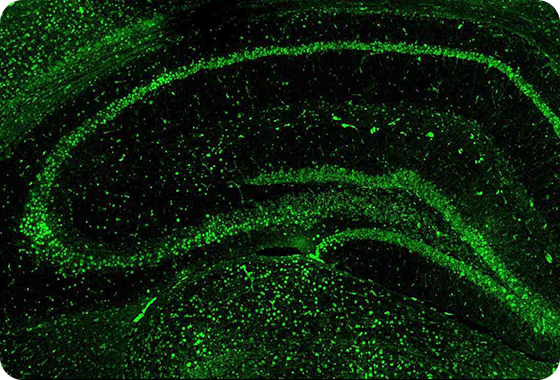
Accumulated of p-Tau in mouse brain
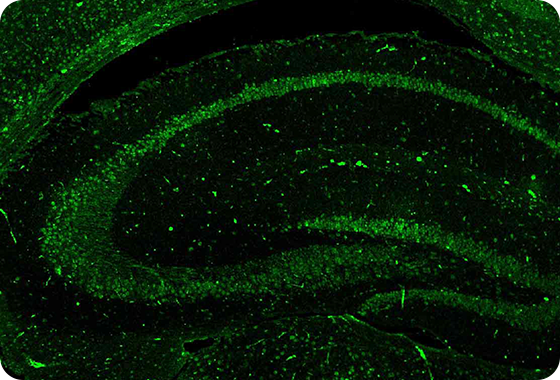
Band of p-Tau is diminished in mouse brain
Reducing amount of p-Tau in Hippocampus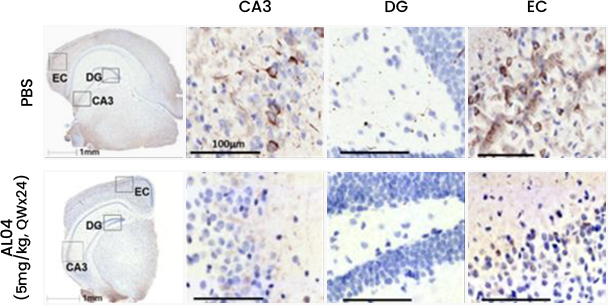
- After administration of AL04, a decrease in hyperphosphorylated Tau and Tau neuro-fibrillary tangles was confirmed in the hippocampus and entorhinal cortex layer of JNPL3 mice through immunohistochemistry

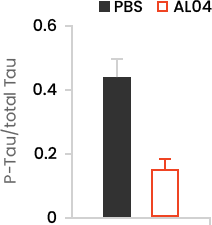
- Through Western blot, a decrease in hyperphosphorylated Tau was confirmed in the entire homogenate of JNPL3 mouse brain parenchyma